September 4, 2014
In today's class, we mainly solved how formulas can be equated to each other to derive other equations.
We were asked to show a pictured explanation of the change in internal energy formula delta E = Q(heat)-W(work). We choose the relationship between the internal energy of the battery and the heat and work caused by the light bulb.\
These equations show how a molecule moves across a certain distance X.
The equation shows that a three-dimensional container with equal speed.
The top and bottom pictures show the change of internal energy of a copper bar. First we calculated the work done to increase the temperature of the bar and we solved for the heat(Q) gained. Since Work and Heat are found we solved for the change in internal energy.
This device is called a fire syringe, which can allow a rapid compression of air in a small glass tube. When the plunger is compressed rapidly, the compressed can be adiabatic, when the surroundings do not exchange heat Q=0.
This is a demonstration of the adiabatic process. Using the fire syringe and a small amount of cotton, we calculated the finally temperature, using the change of space inside the glass tube and the initial temperature.
This shows the calculation to solve the finally temperature inside the glass tube.

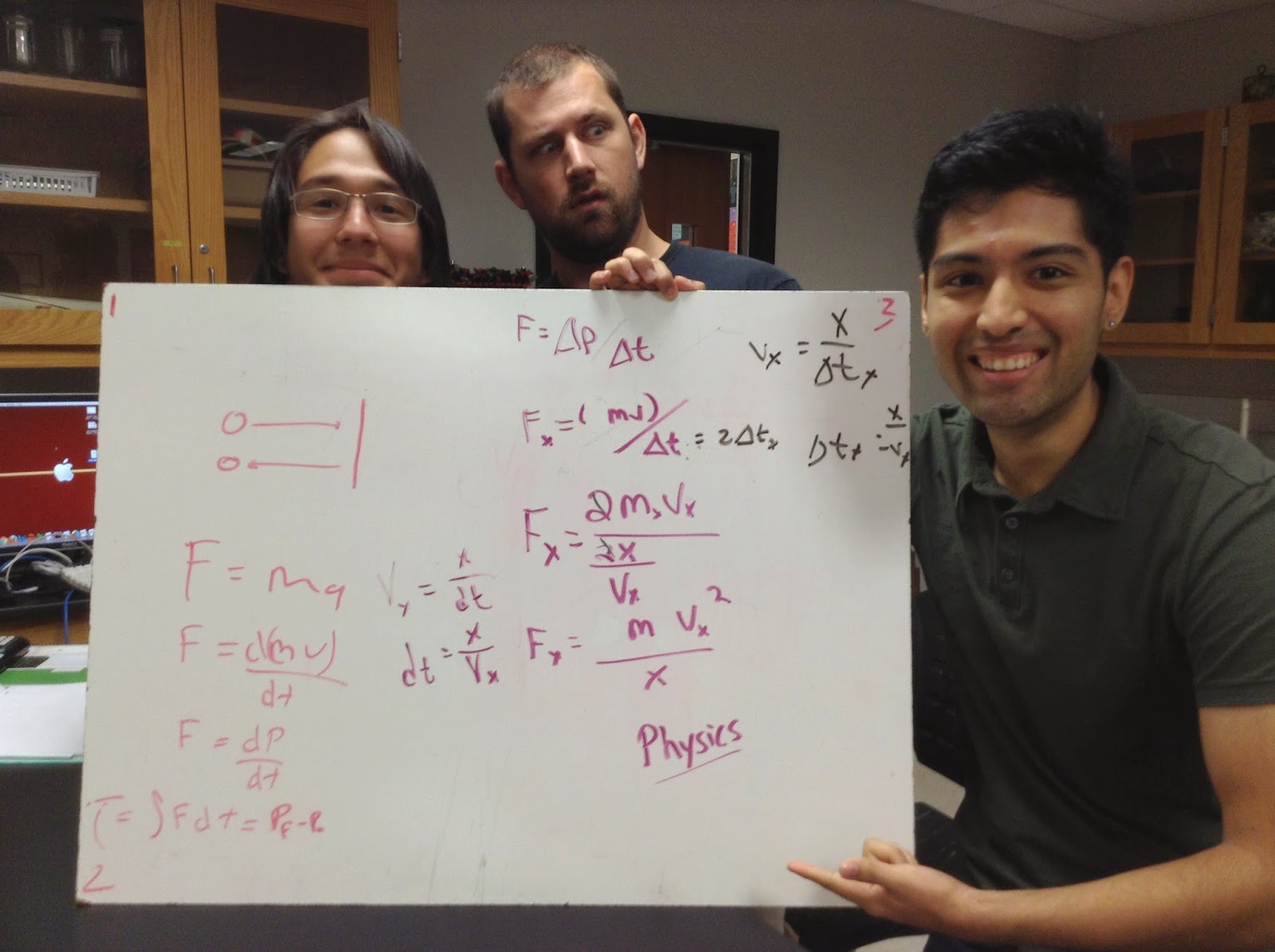






No comments:
Post a Comment