The students executed activities using a manometer to show the effects of changing pressure. The students tested Boyle's law and the professor demonstrated Charles laws and the ideal gas law. The students also solved some questions related to the gas laws.
The professor demonstrated how a heated aluminum can would react to being submerged to water.
The students calculated how much the water moved from its equilibrium when air is introduced at one end of the manometer.
Manometer

The students were asked to predict the graph of Pressure vs Volume.
This pressure sensor was connected to the computer to help graph the Pressure vs Volume.
This graph proved that the relationship between pressure and volume is not linear, which also makes my groups prediction to be wrong.
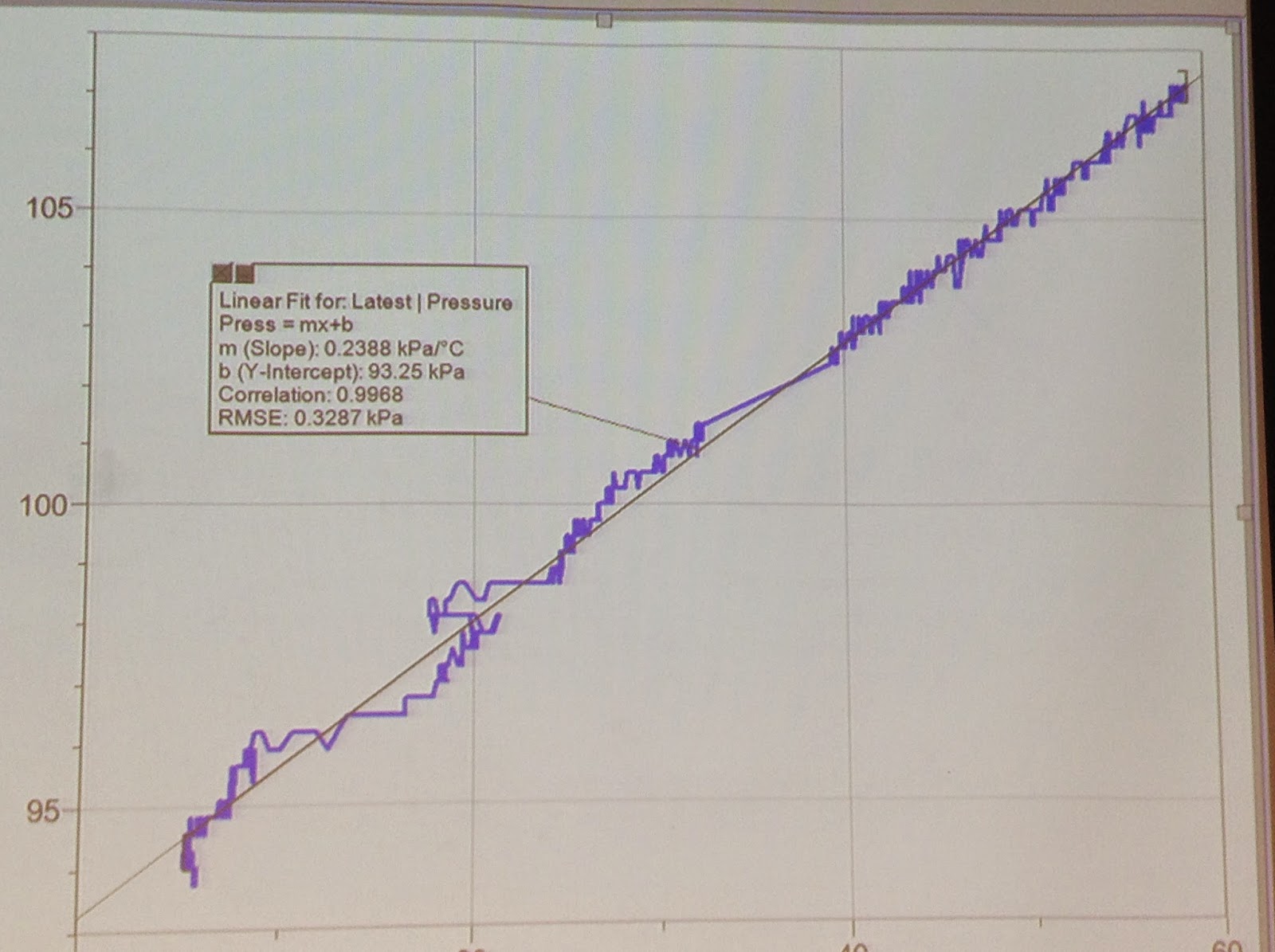
The students were also asked to predict how the graph of Pressure vs Temperature would look like.
A flask is submerged in hot water, which would increase the pressure while temperature also increases.
This graph shows that the relationship between pressure and temperature is linear.
This setup is used to determine the relationship between temperature and volume, by dipping a flask into baths of water with different temperatures.
This graph is the relationship between temperature and volume.
The equation shows the mathematical proof that the equation is linear. And the equation on the bottom left corner of the white broad shows how pressure, volume, and temperature can be related.
This apparatus can decrease the pressure inside the glass dome, and the students can see the effects on a balloon and three marshmallows.
The students were asked to predict the results of decreasing the pressure would affect the marshmallow, and how will marshmallow would react to the pressure returning to normal. The same predictions were also made when asked about how a balloon would react.
The marshmallows increased in size, making the bigger marshmallow to released air from within, and dramatically shrinking from returning the pressure back to normal.
These calculations were used to determine the mass of a balloon released to reach maximum altitude and the initial volume of helium gas.
This is the solution on how much space is left inside the bell while being submerged at a certain distance under water.















No comments:
Post a Comment